Sensing and dynamic measurements
What are biosensors?
The term “biosensor” is short for “biological sensor”, an analytical chemical sensing device which converts a biological response into an electrical signal.
The device is made up of a transducer and a biological element (bioreceptor) that may be an enzyme, an antibody, or a nucleic acid. The bioreceptor interacts with the analyte being tested and the transducer converts the biological response into an electrical, optical, or thermal signal. The final result is a display depicting the presence of the target analyte (Turner et al., 1987).
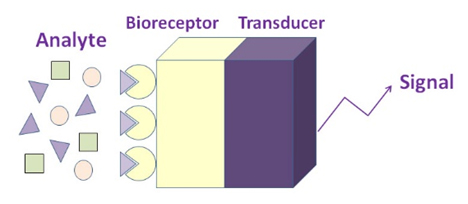
The key element of a biosensor is the transducer, which makes use of a physical change accompanying the reaction. This may be the:
- – heat output by the reaction (calorimetric biosensors)
- – changes in the distribution of charges producing an electrical potential (potentiometric biosensors)
- – movement of electrons produced in a redox reaction (amperometric biosensors)
- – light output or a light absorbance/fluorescence change during the reaction (optical biosensors)
- – effect due to the mass of the reactants or products (piezo-electric biosensors).
A successful biosensor must possess the following beneficial features:
- – the biocatalyst must be highly specific for the purpose of the analyses, be stable under operational conditions, and show good stability over a large number of assays (i.e., >> 100);
- – the reaction should be independent of physical parameters such as stirring, pH, and temperature;
- – the response should be accurate, precise, reproducible, linear, and free from (electrical) noise;
– the complete biosensor should be cheap, small, portable, and capable of being used by semi-skilled operators.
Biosensor applications
The application of biosensors has had a significant impact commercially in a number of areas. The market is comprised of different segments (namely: medical, environmental, and food), with medical applications being the dominant player (Hall, 1986). Biosensors represent a rapidly expanding field, with an estimated 60% annual growth rate and the major impetus coming from the healthcare industry. The estimated world analytical market is about $ 12,000,000,000/year (30% is in the healthcare area): there is clearly a vast market expansion potential as less than 0.1% of this market is currently using biosensors. The demand for reliable, inexpensive, and rapid methods for assessing quality will increase.
Detection of selected D-amino acids (and derivatives)
The presence of D-AAs in biological samples can be detected using different analytical methods, such as capillary gas chromatography, reversed-phase HPLC, and capillary electrophoresis (see above), which often are time-consuming, expensive, and not suitable for on-line application. A rapid and selective determination of D-AAs (and derivatives) may have an important impact on life sciences at different levels. In detail:
– food safety and quality: the total content of D-amino acids is considered a parameter for assessing the quality of food as high levels indicate microbial growth (Rosini et al., 2008);
– environmental sector: the detection of glyphosate, a broad-spectrum herbicide widely used in the world, in water samples is of utmost importance (Pedotti et al., 2009);
– human health: detection of D-aspartate (involved in the pathophysiology of infertility and in the regulation of brain functions) and D-serine (associated with various diseases, such as Alzheimer’s disease, chronic kidney disease, and amyotrophic lateral sclerosis and disorders of the nervous system, such as schizophrenia and bipolar disorder). Among amino acids and amines, glycine [involved in the pathophysiology of schizophrenia and other neurological diseases, see (Rosini et al., 2014a)], histamine [(indicated in the diagnosis of diseases related to its release, the identification of clinical situations, and in defining the mechanisms of adverse reactions to drugs, see (Rosini et al., 2014b)], and sarcosine [(proposed as a novel biomarker for prostate cancer, see (Rosini et al., 2014a)].
Amperometric biosensors
Amperometric biosensors for D-AA detection have been developed based on the detection of hydrogen peroxide or ammonia produced by the D-amino acid oxidase (DAAO) reaction. A chiral analysis of different amino acids was performed by using a composite electrode. DAAO, horseradish peroxidase (HRP), and the mediator ferrocene were coimmobilized by simple physical inclusion into the bulk of a graphite-Teflon electrode matrix (Dominguez et al., 2001). Various immobilization procedures were investigated for the coimmobilization of HRP and DAAO in carbon paste electrodes, using just plain adsorption, adsorption with addition of PEI, glutaraldehyde, or the combination of glutaraldehyde and PEI. A stable response was obtained only by combining adsorption and PEI (0.1%), and a ratio DAAO:HRP 2:1 (Kacaniklic et al., 1994). A rapid measurement of D-AAs was achieved by screen-printed amperometric biosensors incorporating immobilized DAAO and rhodinized carbon to facilitate hydrogen peroxide oxidation (Sarkar et al., 1999). The device responded to all common D-AAs, the exception being D-proline, and exhibited stability over a 56-day period. A homemade bioreactor was built into a FIA system with DAAO immobilized in a thin-layer Plexi cell; the working concentration range was between 0.2 and 3 mM, showing a standard deviation of 2.7% (Varadi et al., 1999). All these sensors showed a broad substrate specificity as they were based on the activity of DAAO from pig kidney, and they do not solve the need for a simple and cheap measuring device.
A quick and selective detection of D-AA content in biological samples was achieved by means of a simple amperometric biosensor using wild-type RgDAAO adsorbed on the graphite electrode (Sacchi et al., 1998). The sensor is characterized by a proportional response between 0.2 and 3 mM D-alanine and shows a linear response starting from 100 µM D-alanine. It is worthy of note that a current response was obtained for the D-amino acids with an acidic side chain when the M213R RgDAAO variant was added in the reaction chamber (Sacchi et al., 2004). The total D-AA content in food specimens was assayed by an inexpensive, simple, and rapid device in which the D-amino acid composition does not alter the results. A screen-printed electrode amperometric biosensor based on a mixture of RgDAAO variants was used; the response was independent of the composition of the solution, with a detection limit of 0.25 mM and a mean response time of 10-15 min (Rosini et al., 2008). Notably, a microbiosensor based on a cylindrical platinum microelectrode covered with a membrane of poly-m-phenylenediamine and a layer of immobilized DAAO from Rhodotorula gracilis (RgDAAO) was developed to monitor D-serine levels in vivo (Pernot et al., 2008); a detection limit of 16 nM and a mean response time of 2 s were achieved.
Fluorimetric assays
In this context, two biosensors based on different, selected enzyme variants of the flavoenzyme glycine oxidase were set up. The system is based on a simple fluorimetric assay, characterized by low cost and ease of use; the fluorescence intensity emission of a commercial dye transducer is proportional to the analyte concentration (Rosini et al., 2014a). In real time, the optical sensing system assays glycine or sarcosine in biological samples with a detection limit ≤ 0.5 µM. The glycine concentration detected in U87 human glioblastoma cell extracts is in good agreement with the value obtained by using the reference HPLC method (7.5 versus 6.7 µM, respectively); interestingly, the glycine concentration was quantified in human plasma using this assay and the results were in good agreement with the values reported in the literature.
References
Domínguez R., Serra B., Reviejo A.J., Pingarrón J.M. Chiral analysis of amino acids using electrochemical composite bienzyme biosensors (2001) Anal. Biochem., 298: 275-282
Hall E.A.H., The developing biosensor arena (1986) Enz. Microb. Technol., 8: 651-658
Kacaniklic V., Johansson K., Marko-Varga G., Gorton L., Jönsson-Pettersson G., Csöregi E., Amperometric biosensors for detection of L- and D-amino acids based on coimmobilized peroxidase and L- and D-amino acid oxidases in carbon paste electrodes (1994) Electroanalysis, 6: 381-390
Pedotti M., Rosini E., Molla G., Moschetti T., Savino C., Vallone B., Pollegioni L., Glyphosate resistance by engineering the flavoenzyme glycine oxidase (2009) J. Biol. Chem., 284: 36415-36423
Pernot P., Mothet J.P., Schuvailo O., Soldatkin A., Pollegioni L., Pilone M., Adeline M.T., Cespuglio R., Marinesco S., Characterization of a yeast D-amino acid oxidase microbiosensor for D-serine detection in the central nervous system (2008) Anal. Chem., 80: 1589-1597
Rosini E., Molla G., Rossetti C., Pilone M.S., Pollegioni L., Sacchi S., A biosensor for all D-amino acids using evolved D-amino acid oxidase (2008) J. Biotechnol., 135: 377-384
Rosini E., Piubelli L., Molla G., Frattini L., Valentino M., Varriale A., D’Auria S., Pollegioni L., Novel biosensors based on optimized glycine oxidase (2014a) FEBS J., 281: 3460-3472
Rosini E., Tonin F., Vasylieva N., Marinesco S., Pollegioni L. “Evolution of histamine oxidase activity for biotechnological applications” (2014b) Appl. Microbiol. Biotechnol., 98: 739-748
Sacchi S., Pollegioni L., Pilone M.S., Rossetti C., Determination of D-amino acids using a D-amino acid oxidase biosensor with spectrophotometric and potentiometric detection (1998) Biotechnol. Tech., 12: 149-153
Sacchi S., Lorenzi S., Molla G., Pilone M.S., Rossetti C., Pollegioni L., Engineering the substrate specificity of D-amino-acid oxidase (2004) J. Biol. Chem., 277: 27510-27516
Sarkar P., Tothill I.E., Setford S.J., Turner A.P., Screen-printed amperometric biosensors for the rapid measurement of L- and D-amino acids (1999) Analyst, 124: 865-870
Turner A.P.F., Karube I., Wilson G.S. “Biosensors: fundamentals and applications” (1987) Oxford, U.K.: Oxford University Press
Váradi M., Adányi N., Szabó E.E., Trummer N., Determination of the ratio of D- and L-amino acids in brewing by an immobilised amino acid oxidase enzyme reactor coupled to amperometric detection (1999) Biosens. Bioelectron., 14: 335-340
Author
Elena Rosini, Università degli studi dell’Insubria
